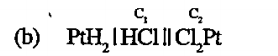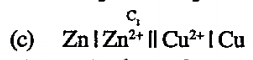A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MBD PUBLICATION-ELECTROCHEMISTRY-QUESTION BANK
- A fuel cell is :
Text Solution
|
- The standard reduction potential values of three metallic cations X, Y...
Text Solution
|
- Which represents a concentration cell:
Text Solution
|
- Aqueous solution of HCI conducts electricity because :
Text Solution
|
- When an electrolyte solution conducts electricity, current is carried ...
Text Solution
|
- The reaction taking place at anode when an aqueous solution of CuSO4 i...
Text Solution
|
- In the electrolysis of which solution, OH^- ions are discharged in pre...
Text Solution
|
- Which reaction occurs at cathode during electrolysis of fused lead bro...
Text Solution
|
- The process in which chemical change accompanies the passage of curren...
Text Solution
|
- Which is correct about fuel cells :
Text Solution
|
- Calculate the volume of hydrogen at NTP obtained by passing a current ...
Text Solution
|
- During electrolysis of aqueous solution of NaCl at which electrode chl...
Text Solution
|
- At 25^@C, the standard emf of cell having reactions involving two elec...
Text Solution
|
- The standard reduction potentials of Cu^(2+)/Cu and Cu^(2+)/Cu^+ are 0...
Text Solution
|
- The emf of the cell, Zn|Zn^(2+)(1M)||Cu^(2+)|Cu(1M) is 1.1 volt, if ...
Text Solution
|
- For I2+2erarr2I^-, standard reduction potential =+0.54 volt. For Br^(...
Text Solution
|
- For the cell prepared from electrode A and B: Electrode A:Cr2O7^(2-)|C...
Text Solution
|
- The following facts are available :2A^- +B2 rarr2B^- +A2,2C^-+B2rarrNo...
Text Solution
|
- Given that E(Fe^3+|Fe) and E(Fe^2|Fe)^@ are -0.36V and-0.439V, respect...
Text Solution
|
- The standard oxidation potentials of the electrodes Ag|Ag^+,Sn|Sn^(2+)...
Text Solution
|