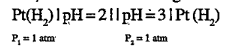A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MBD PUBLICATION-ELECTROCHEMISTRY-QUESTION BANK
- A hydrogen electrode placed in a buffer solution of CH3COONa and aceti...
Text Solution
|
- The amount of energy expanded during the passage of one ampere current...
Text Solution
|
- The cell reaction for the given cell is :Pt(H2)|pH=2||pH=3|Pt(H2)
Text Solution
|
- The cell reaction for the given cell is spontaneous if :Pt(H2)|H^=(1M)...
Text Solution
|
- The cell reaction for the given cell is spontaneous if :Pt | Cl2|CI^(-...
Text Solution
|
- Passage of threee faraday of charge through aqueous solution of AgNO3,...
Text Solution
|
- The approximate emf of a dry cell is :
Text Solution
|
- Which gains electrons more easily :
Text Solution
|
- Which will increase the voltage of the cell Sn(s) +2Ag^+(aq)rarr Sn^(...
Text Solution
|
- During electrolysis of fused CaH2,H2 is liberated at :
Text Solution
|
- Which defines the standard reduction electrode potential of Zn^(2+) io...
Text Solution
|
- In which cell, electrical energy is converted into chemical energy:
Text Solution
|
- E^@ for Fe^(2+) +2e rarrFe is -0.44 volt and E^@ for Zn^(2+) +2e rarrZ...
Text Solution
|
- In a galvanic cell, which is wrong :
Text Solution
|
- Using same quantity of current , which among Na, Mg and AI is deposite...
Text Solution
|
- A metal having negative reduciton potential when dipped in the solutio...
Text Solution
|
- In electrochemical corrosion of metals, the metal undergoing corrosion...
Text Solution
|
- The metal which cannot liberate H2 from hydrochloric acid is :
Text Solution
|
- KCl (aq) cannot be used as a salt bridge for the cell Cu(s)|CuSO4(aq)|...
Text Solution
|
- The calomel electrode is reversible with respect to:
Text Solution
|