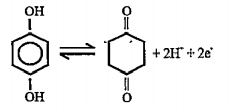
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MBD PUBLICATION-ELECTROCHEMISTRY-QUESTION BANK
- A solution containing one mole per litre each of Cu(NO3)2, AgNO3, Hg2(...
Text Solution
|
- In a galvanic cell energy changes occurs as:
Text Solution
|
- AtpH=2,E("Quinhydrone")^@ =1.30V,E("Quinhydrone") will be:
Text Solution
|
- The passage of electricity in the Daniell cell when Zn and Cu electrod...
Text Solution
|
- A correct electrochemical series can be obtained from K,Ca,Na,Al,Mg,Zn...
Text Solution
|
- Indicator electrode is :
Text Solution
|
- When Zn piece is kept inCuSO4 solution copper gets prectipitated becau...
Text Solution
|
- The standard oxidation potentials of Zn and Ag in water at 25^@ C are....
Text Solution
|
- The standard reduction potentials of the elemtnts A, B.Care +2.37V,-1....
Text Solution
|
- Is the reaction, 2Al +3Fe^(2+)rarr2Al^(3+) +3Fe possible?
Text Solution
|
- Whether tin can displace lead from aqueous lead bromide solution:
Text Solution
|
- Faraday is equal to :
Text Solution
|
- More electropositive elements have :
Text Solution
|
- Based on the data given below , the correct order of reducing power is...
Text Solution
|
- Galvanised iron sheets have coating of :
Text Solution
|
- The electrochemical that is easiest to be reduced is :
Text Solution
|
- An electrochemical cell consists of :
Text Solution
|
- The correct order of chemical reactivity with water according to elect...
Text Solution
|
- Which graph correctly correlates E(cell) as a function of concentratio...
Text Solution
|
- Faraday.s laws hold good at:
Text Solution
|