A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MBD PUBLICATION-ELECTROCHEMISTRY-QUESTION BANK
- An electrochemical cell consists of :
Text Solution
|
- The correct order of chemical reactivity with water according to elect...
Text Solution
|
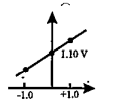
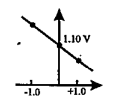
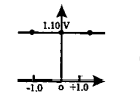
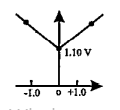
- Which graph correctly correlates E(cell) as a function of concentratio...
Text Solution
|
- Faraday.s laws hold good at:
Text Solution
|
- The main function of the salt bridge is:
Text Solution
|
- A substance that will reduce Ag^+ to Ag but will not reduce Ni^(2+) to...
Text Solution
|
- A dilute aqueous solution of Li2SO4 is electrolysed . The products for...
Text Solution
|
- Blocks of magnesium metal are often strapped to the steel hulls of oce...
Text Solution
|
- Which statement is true about spontaneous cell reaction in galvanic ce...
Text Solution
|
- It is impossible to measure the actual voltage of any half cell by its...
Text Solution
|
- Which metal will dissolve if the cell works Cu|Cu^(2+)||Ag^+|Ag:
Text Solution
|
- In the concentration cells the electrical energy is produced due to :
Text Solution
|
- The Zn acts as sacrificial or cathodic porection to prevent rusting of...
Text Solution
|
- The number of faraday required to liberate 1 mole of any element indic...
Text Solution
|
- Quantity of electricity is measured in :
Text Solution
|
- Which are used as secondary reference electrodes :
Text Solution
|
- The corrosion of iron object is favoured by:
Text Solution
|
- For a redox reaction to proceed spontaneously in a given direction, th...
Text Solution
|
- In a cell containing zinc electrode and standard hydrogen electrode(SH...
Text Solution
|
- A cell in which electric current is produced by net oxidation and redu...
Text Solution
|