A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MBD PUBLICATION-CHEMICAL KINETICS-QUESTION BANK
- The chemical reaction, 2O3rarr3O2 proceeds as follows: O3iffO2+..........
Text Solution
|
- A hypothetical reaction, A2+B2rarr2AB follows the mechanism as given b...
Text Solution
|
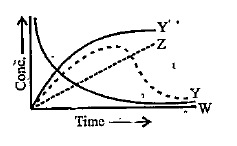
- For the reaction ,A+BrarrC+D. The variation of the concentration of th...
Text Solution
|
- If the first order reaction involves gaseous reactants and gaseous-pro...
Text Solution
|
- The branch of chemistry which deals with the reaction rates and. react...
Text Solution
|
- For an exothermic chemical process occuring in two steps as :A+BrarrX(...
Text Solution
|
- Among the following reaction the fastest one is :
Text Solution
|
- In acidic medium the rate of reaction between(BrO3)^- and Br^- ions is...
Text Solution
|
- Chemical reaction occurs as a result of collisions between reacting mo...
Text Solution
|
- The activation energies of two reactions are Ea andEawith Eagt Ea if ...
Text Solution
|
- For the decomposition of N2O5(g), it is given that :2N2O5(g)rarr4NO2(g...
Text Solution
|
- For the reaction:[Cu(NH3)4]^(2+)+H2Orarr[Cu(NH3)3H2O^(2++)NH3the net r...
Text Solution
|
- Which is correct relation in between (dC)/dt,(dn)/dtand(dP)/dtwhere C,...
Text Solution
|
- Rate of a reaction :
Text Solution
|
- The dimensions of the rate constant of a second order reaction involve...
Text Solution
|
- The rate constant is given by the equation K=Ae^(-Ea//RT)which factor ...
Text Solution
|
- For the reaction ,H2(g)+Br(g)=2HBr(g),the reaction rate=K[H2][Br2]^1/2...
Text Solution
|
- Which curve represents zero order reaction:
Text Solution
|
- Rate equation for a second order reaction is :
Text Solution
|
- In Arrhenius equation K=Ae^-Ea//RT ,the quantity-Ea//RT is referred as...
Text Solution
|