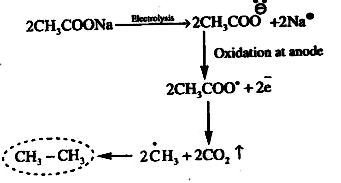A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- When aqueous solution of sodium ethanoate electrolysed, the product(s)...
Text Solution
|
- When aqueous of sodium ethanote is electrolysed, the product(s) at ano...
Text Solution
|
- When an aqueous solution of H(2)SO(4) is electrolysed, the ion dischar...
Text Solution
|
- An aqueous solution of sodium sulphate is electrolysed using inert ele...
Text Solution
|
- When an aqueous solution of sodium propionate is electrolysed the gas ...
Text Solution
|
- What are the electrolysed product when aqueous sodium chioride is elec...
Text Solution
|
- When an aqueous solution of KBr is electrolysed, Br(2) is evolved at a...
Text Solution
|
- An aqueous solution of sodium sulphate is electrolysed using inert ele...
Text Solution
|
- What are the products formed at anode and cathode when aqueous solutio...
Text Solution
|