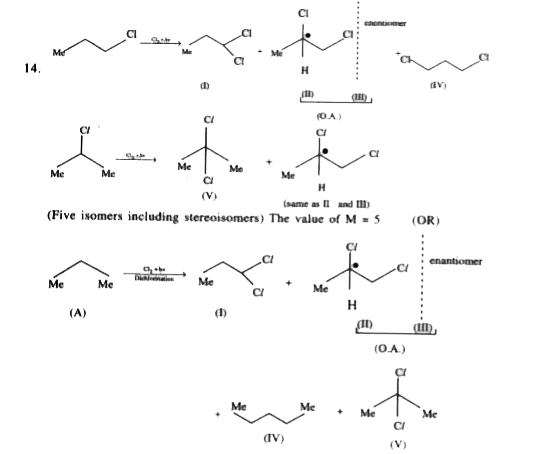
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Fifteen milliliters of gaseous hydrocarbon (A) was required for comple...
Text Solution
|
- 50 " mL of " a gaseous hydrocarbon A requried for complete combustion....
Text Solution
|
- Fifteen milliliters of gaseous hydrocarbo (A) was required for complet...
Text Solution
|
- 15mL of a gaseous hydrocarbon required for complete combustion, 357mL ...
Text Solution
|
- Twenty millilitres of a gaseous hydrocarbon required 400 ml of air for...
Text Solution
|
- Twenty millilitres of a gaseous hydrocarbon required 400 ml of air for...
Text Solution
|
- Twenty millilitres of a gaseous hydrocarbon required 400 ml of air for...
Text Solution
|
- Twenty millilitres of a gaseous hydrocarbon required 400 ml of air for...
Text Solution
|
- Twenty millilitres of a gaseous hydrocarbon required 400 ml of air for...
Text Solution
|