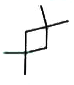
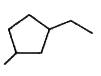
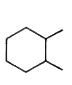
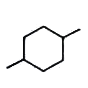
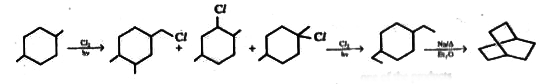
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A hydrocarbon P has molecular formula C8H(16), and it does not undergo...
Text Solution
|
- An alkane with the molecular formula C(6)H(14) forms only three monoch...
Text Solution
|
- Three structures P,Q and R having seme molecular formula, i.e., C(5)H(...
Text Solution
|
- Among the isomeric alkanes formula C(5)H(12) , identify the one that o...
Text Solution
|
- An organic compound P has molecular formula C(2)H(4). On reduction, it...
Text Solution
|
- C(5)H(12) अणुसूत्र वाले समावयवी एल्केनों में से उसको पहचानिये जो प्रका...
Text Solution
|
- C(5)H(12) अणुसूत्र वाले समावयवी एल्केनों में से उसको पहचानिये जो प्रका...
Text Solution
|
- C(5)H(12) अणुसूत्र वाले समावयवी एल्केनों में से उसको पहचानिये जो प्रका...
Text Solution
|
- Write down the structure of a hydrocarbon (molecular formula: C(6)H(12...
Text Solution
|