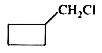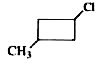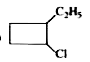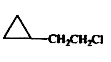A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
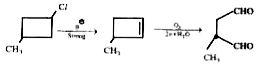
- A compound "X" has molecular formula C5H(9) Cl. It does not react with...
Text Solution
|
- When a compound X reacts with ozone in aq medium, a compound Y is prod...
Text Solution
|
- A compond A has the molecular formula C5H9Cl.It does not react with b...
Text Solution
|
- A hydrocarbon (X) of the formula C6H12 does not react with bromine wat...
Text Solution
|
- A compound A has the molecular formula C(5)H(9)Cl. It does not react w...
Text Solution
|
- A compound (A) has molecular formula C(5)H(9)CI It does not react with...
Text Solution
|
- A compound (A) has molecular formula C(5)H(9)CI It does not rea...
Text Solution
|
- A compound (A) has molecular formula C(5)H(9)CI It does not rea...
Text Solution
|
- Compounds having molecular formula C5H(12)O may be classified as .
Text Solution
|