A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
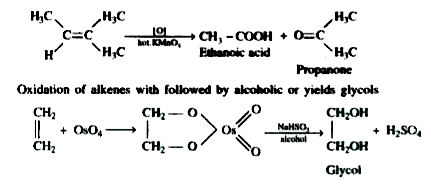
- Oxidation of alkenes by cleavage with acidic or alkaline KMnO4 or acid...
Text Solution
|
- Which alkene on oxidation with acidic KMnO(4) gives only acetic acid?
Text Solution
|
- Oxidation of alkenes by cleavage with the acidic or alkaline KmNO(4) o...
Text Solution
|
- Oxidation of alkenes by cleavage with the acidic or alkaline KmNO(4) o...
Text Solution
|
- Oxidation of alkenes by cleavage with the acidic or alkaline KmNO(4) o...
Text Solution
|
- Oxidation of alkenes by cleavage with the acidic or alkaline KmNO(4) o...
Text Solution
|
- Oxidation of alkenes by cleavage with the acidic or alkaline KmNO(4) o...
Text Solution
|
- Give example of an alkene which on oxidation by acidic solution of KM...
Text Solution
|
- An alkene on vigorous oxidation with KMnO(4) gives only propionic acid...
Text Solution
|