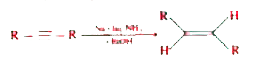A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In the conversion of alkyne to trans -alkene by birch reduction using ...
Text Solution
|
- Terminal alkynes (RC-=CH) are not reduced by alkali metals (e.g., Na, ...
Text Solution
|
- In the conversion of alkyne to trans-alkene by Birch reduction using a...
Text Solution
|
- The trans-alkenes are formed by the reduction of alkynes with :
Text Solution
|
- The trans-alkenes are formed by the reduction of alkynes with
Text Solution
|
- The trans-alkenes are formed by the reduction of alkynes with
Text Solution
|
- The trans-alkenes are formed by the reduction of alkynes with :
Text Solution
|
- Reduction of Alkyne (Birch & Lindlars)
Text Solution
|
- The trans-alkenes are formed by the reduction of alkynes with-
Text Solution
|