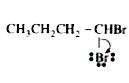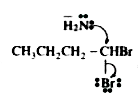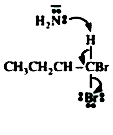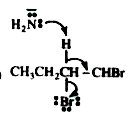A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- 1) The -=C-H unit of an alkyne is more acidic than a C – H unit of an ...
Text Solution
|
- S-I: Alkynes are more reactive than alkene towards catalytic hydrogena...
Text Solution
|
- ऐल्केन , ऐल्कीन तथा अल्काइन में C की संकरण अवस्था बताइए ।
Text Solution
|
- ऐल्केन एल्कीन तथा एल्काइन किसे कहते है।
Text Solution
|
- Alkenes and alkynes are unsaturated hydrocarbons and undergo electroph...
Text Solution
|
- Alkenes and alkynes are unsaturated hydrocarbons and undergo electroph...
Text Solution
|
- Alkenes and alkynes are unsaturated hydrocarbons and undergo electroph...
Text Solution
|
- Alkenes and alkynes are unsaturated hydrocarbons and undergo electroph...
Text Solution
|
- Alkynes are .......... acidic than alkenes.
Text Solution
|