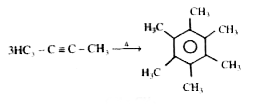
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Aromaticity in benzene is due to
Text Solution
|
- Which of the followig is aromatic like benzene?
Text Solution
|
- Aromatic character of benzene can be explained by
Text Solution
|
- Aromatic properties of benzene are proved by
Text Solution
|
- Benzene is an aromatic compound. True/false
Text Solution
|
- Hydrocarbons - Aromatic - Benzene
Text Solution
|
- Aromatic compound| alkyl benzene|benzaldehyde|aromatic ketones|
Text Solution
|
- बेंजीन का एरोमैटिक गुण किसके द्वारा सिद्ध होता है ?
Text Solution
|
- बेंजीन और इसके व्युत्पन्नों (एरोमैटिक हाइड्रोकार्बन्स) की सामान्य क्रि...
Text Solution
|