A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Hexachloro benzene is obtained by addition of 'x' moles of Cl2 with C6...
Text Solution
|
- Adding of Cl2 to benzene in the presence of AlCl3 is an example of
Text Solution
|
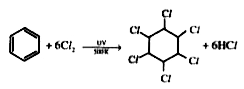
- In presence of UV light, benzene reacts with chlorine forming
Text Solution
|
- बेंजीन में किसी योगिक के X मोलल विलयन में विलेय का मोल प्रभाज 0.2 है. ...
Text Solution
|
- Benzene is treated with methyl chloride in presence of anhydrous AlCl3...
Text Solution
|
- Benzene reacts with Cl2 in the presence of FeCl3 and in absence of su...
Text Solution
|
- Benzene reacts with Cl2 in the presence of sunlight to give....
Text Solution
|
- (i) क्लोरोबेंजीन और (ii) हैक्साक्लोरो साइक्लोहैक्सेन बेंजीन पर क्लोरीन...
Text Solution
|
- One mole Benzene in presence of light on reaction with 'x' moles of Cl...
Text Solution
|