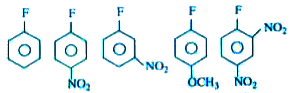A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Ease of aromatic nucleophilic substitution among these compound will b...
Text Solution
|
- Aromatic Nucleophilic Substitution Reaction
Text Solution
|
- Which of the following compound is most reactive in the nucleophilic a...
Text Solution
|
- Identify the compounds that will undergo nucleophilic aromatic substit...
Text Solution
|
- Order of reactivity towards nucleophilic substitution reaction of the ...
Text Solution
|
- Eacof nucleophilic substitution among these compounds will be in the o...
Text Solution
|
- What is the order of nucleophilic substitution in the aromatic ring
Text Solution
|
- What is the order of nucleophilic substitution in the aromatic ring
Text Solution
|
- Alkanes undergo free radical substitution, aromatic compounds undergo ...
Text Solution
|