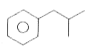
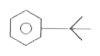
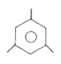
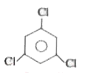
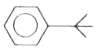
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Benzene undergoes both Friedel-Crafts alkylation and acylation reactio...
Text Solution
|
- Assertion: Friedel-Crafts reaction is used to introduce an alkyl or ac...
Text Solution
|
- Assertion : Alkyl benzene is not prepared by Friedel- Crafts alkylatio...
Text Solution
|
- Benzene under goes electrophilic substitution reactions like nitration...
Text Solution
|
- Assertion:Alkyl benzene is not prepared by Friedel-Crafts alkylation o...
Text Solution
|
- Assertion. Friedel-Crafts reaction is used to introduce an alkyl or ac...
Text Solution
|
- Assertion. Alkylbenzene is not prepared by Friedel-Crafts alkylation o...
Text Solution
|
- Why is Friedel-Crafts acylation of benzene is favourable than that of ...
Text Solution
|
- Name reactions : Friedel Crafts alkylation and acylation.
Text Solution
|
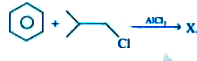 , Compound X is
, Compound X is