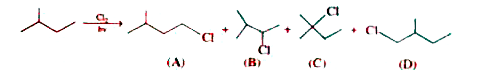
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The relative reactivity of 1^(@), 2^(@) and 3^(@) hydrogens in chlorin...
Text Solution
|
- The relative reactivity of 1^(@)H,2^(@)H and 3^(@)H in bromination rea...
Text Solution
|
- The relative reactivity of 1^(@)H,2^(@)H and 3^(@)H in bromination rea...
Text Solution
|
- The relative reactivity of 1^(@), 2^(@) and 3^(@) hybrogen's towards c...
Text Solution
|
- The relative reactivity of 1^@,2^@,3^@ hydrogen's towards chlorination...
Text Solution
|
- अभिक्रिया A+B + C to Products के लिए अभिक्रिया की दर निम्न है- -(d[...
Text Solution
|
- If the reactivity factor for chlorine substitution through free radica...
Text Solution
|
- Predict the percentage of isomers formed during monobromination of 2,3...
Text Solution
|
- Calculate the percentages of all the monochlorinated products obtained...
Text Solution
|