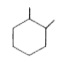
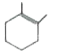
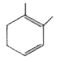
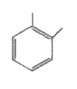
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- n-Octane in presence of Cr2O3 // Al2O3 ultimately changes into
Text Solution
|
- The value of triangle(f)G^(ɵ)(Cr2O3)=-540kJmol^-1 and triangle(f)G^(ɵ)...
Text Solution
|
- Aromatisation of n-heptane by passing over (Al2O3 + Cr2O3) catalyst at...
Text Solution
|
- Catalytic dehydrogenation of n-heptane in presence of Cr2O3//Al2O3 at ...
Text Solution
|
- n-Octane when heated to 773 K under a pressure of 10-20 atm and in pre...
Text Solution
|
- Which of the following products is formed when n - heptane is passed o...
Text Solution
|
- Which of the following products is formed when n - heptane is passed o...
Text Solution
|
- The enthalpies of formation of Al2O3 and Cr2O3 are -1596 kJ and -1134 ...
Text Solution
|
- Al2O3 And Cr2O3 If the enthalpy values of -1596 KJ and -1134 KJ, respe...
Text Solution
|