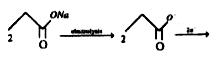A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Alkanes can be prepared from sodium (or) potassium salts of carboxylic...
Text Solution
|
- Which of the following alkanes cannot be produced by Kolbe's electroly...
Text Solution
|
- In Kolbe's electrolysis method, electrolysis of aquesous solution of s...
Text Solution
|
- For preparing an alkane , a concentrated aqueous solution of sodium or...
Text Solution
|
- For preparing an alkane , a concentrated aqueous solution of sodium or...
Text Solution
|
- For the preparation of alkanes ,a saturated solution sodium o...
Text Solution
|
- How can cyclobutane be prepared by Kolbe's electrolysis method ?
Text Solution
|
- Which alkane cannot be prepared by Kolbe's method ?
Text Solution
|
- Which can be prepared by Kolbe's electrolysis?
Text Solution
|