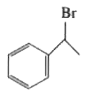
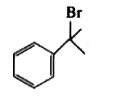
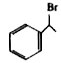
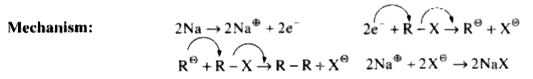A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Alkane may be prepared from alkyl halide by Wurtz method where alkyl h...
Text Solution
|
- The reactivity of alkyl halides for Wurtz reaction is:
Text Solution
|
- In Wurtz reaction alkyl halide react with
Text Solution
|
- The reactivity of alkyl haliders for Wurtz reaction is :
Text Solution
|
- Alkane may be prepared from alkyl hlide by Wutrtz method where alkyl h...
Text Solution
|
- Alkane may be prepared from alkyl hlide by Wutrtz method where alkyl h...
Text Solution
|
- Alkane may be prepared from alkyl hlide by Wutrtz method where alkyl h...
Text Solution
|
- In Wurtz reaction of alkyl halides, the reactivity of alkyl halides fo...
Text Solution
|
- 2R - X + 2Na overset("dry ether") rarr R - R + 2Nax Which of the follo...
Text Solution
|