A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Oxymercuration demercuration reacti on is process of addition H2O acco...
Text Solution
|
- Oxymercuration demercuratioon reaction is process of additon H(2)O acc...
Text Solution
|
- The product of following reaction is CH3-undersetunderset(CH3)|overset...
Text Solution
|
- The product Z in the following series of reaction is : CH3CH2 -overset...
Text Solution
|
- निम्न क्रिया का उत्पाद होगा CH3 - underset(CH3) underset(|) overset(...
Text Solution
|
- CH3 - overset(CH3)overset(|)C = CH-CH3 overset(Hg(OAc)2 // EtOH)to und...
Text Solution
|
- Oxymercuration demercuration reacti on is process of addition H2O acco...
Text Solution
|
- Oxymercuration demercuration reacti on is process of addition H2O acco...
Text Solution
|
- Oxymercuration demercuration reacti on is process of addition H2O acco...
Text Solution
|
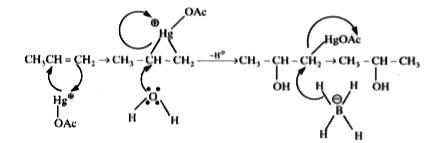
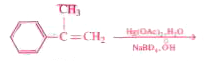 Major product :
Major product :