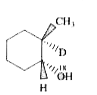
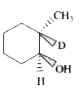
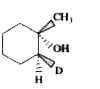
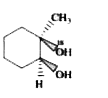
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
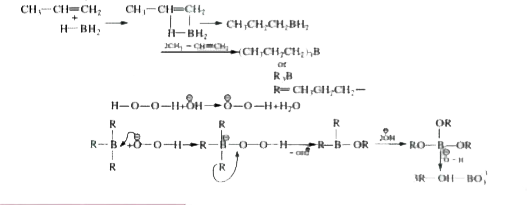
- Hydroboration oxidation reaction is process of addition of H2O accordi...
Text Solution
|
- Hydroboration oxidation reaction is a process of addtition of H(2)O ac...
Text Solution
|
- Hydroboration oxidation reaction is a process of addtition of H(2)O ac...
Text Solution
|
- In the reaction CH3CH=CH2+H2O + [O] underset"Acid"overset(KMnO4)to ...
Text Solution
|
- The give reaction H-underset(CH3)underset(|)overset(CH2-CH3)overset(|)...
Text Solution
|
- Complete the following reaction CH3-CH2-CH2- undersetoverset(||)(O)(C ...
Text Solution
|
- CH3 - overset(Cl)overset(|) CH -CH3 overset(Alc.KOH)to CH3 -CH =CH2 ...
Text Solution
|
- Hydroboration oxidation reaction is process of addition of H2O accordi...
Text Solution
|
- CH3 - CH2-CH2 -C -= CH underset(THF)overset(BH3)to underset(OH^(-))ove...
Text Solution
|
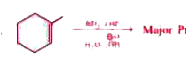 Major product
Major product