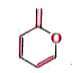A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- How many "pi" (pi) electrons are there in the following molecule?
Text Solution
|
- How many pi electrons are there in the following species ?
Text Solution
|
- How many pi electrons are contained in each molecule ?
Text Solution
|
- How many pi electrons are there in
Text Solution
|
- How many sigma and pi bonds are in the molecule of tetracyanoethylene
Text Solution
|
- How many pi electron are there in the following speices :
Text Solution
|
- One pi bond corresponds to how many pi electrons
Text Solution
|
- निम्न यौगिक में कितने विस्थापनीय pi-इलेक्ट्रॉन हैं?
Text Solution
|
- how many pi electrons are there in the following species ?
Text Solution
|