A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- For the reaction, reactants . The bset combination of reactant is
Text Solution
|
- The reactant (A) is the reaction is : .
Text Solution
|
- Reactant A of the above reaction is :
Text Solution
|
- Reactant (A) in this reaction is :
Text Solution
|
- For the reaction, ? , the best combination of reactants is:
Text Solution
|
- In endothermic reactions the reactants :
Text Solution
|
- उस अभिक्रिया का नाम बताएँ जिसमे दो या अधिक अभिकारक परस्पर संयोग करके ए...
Text Solution
|
- reactant is the reactant that reacts completely but limits further pro...
Text Solution
|
- A reaction in which two or more reactants combine to form a compound i...
Text Solution
|
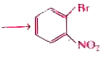 . The bset combination of reactant is
. The bset combination of reactant is