A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
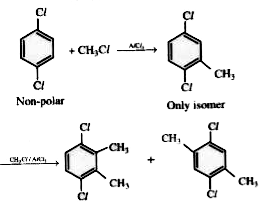
- Consider the following reaction . C6H4 Cl2 (X) + CH3Cl overset(AlCl3...
Text Solution
|
- Select the correct statement about the following reaction CH3-under...
Text Solution
|
- निम्नलिखित अभिक्रिया क्रम में, फीनॉल overset("Zn रज")to X underset...
Text Solution
|
- A + B to CH3 -OC (CH3 ) overset( HI) to X+ Y Correct statement among t...
Text Solution
|
- C6H6+CH3Cl overset(AlCl3)(to) + C6H5CH3 + HCl . The reaction is known ...
Text Solution
|
- The reaction C6H6 + CH3Cl underset("(anhydrous)")overset(AlCl3)(rarr) ...
Text Solution
|
- What are X and Y respectively in the following reaction? Z - product...
Text Solution
|
- Consider the following reaction . C6H4 Cl2 (X) + CH3Cl overset(AlCl3...
Text Solution
|
- What are X and Y respectively in the following reaction? Z - product...
Text Solution
|