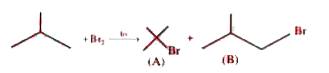A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The relative of 1^@ h, 2H^@ and 3^@ H in bromination reaction h...
Text Solution
|
- Calculate the percentage of compounds obtained by monobromination of i...
Text Solution
|
- The relative reactivity of 1^(@)H,2^(@)H and 3^(@)H in bromination rea...
Text Solution
|
- The relative reactivity of 1^(@) : 2^(@) : 3^(@) H's to brominate is ...
Text Solution
|
- The relative reactivity of 1^(@)H,2^(@)H and 3^(@)H in bromination rea...
Text Solution
|
- The relative reactivity of 1^(@),2^(@) and 3^(@) hydrogens in chlorina...
Text Solution
|
- A certain reaction is at equilbrium at 82^(@)C and Delta H for this re...
Text Solution
|
- The relative of 1^(@):2^(@):3^(@) hydrogen to bromination is 1:8...
Text Solution
|
- Calculate the % yield of monochloro products when isopentane is chlori...
Text Solution
|