A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The rate of nitration will be
Text Solution
|
- The rate of nitration of phenol
Text Solution
|
- Compare the rate of reaction of following for nitration reaction.
Text Solution
|
- Which of the following compounds undergoes nitration at fastest rate...
Text Solution
|
- Arrange the following in incresing order of rate of Nitration : (a)
Text Solution
|
- In nitration of benzene by mixed acid the rate of reaction will be:
Text Solution
|
- Arrange the following in decreasing order of rate of nitration :-
Text Solution
|
- Rate determining step in nitration of benzene is
Text Solution
|
- Consider the nitration of benzene using mixed conc.H2SO4 and HNO3 . If...
Text Solution
|
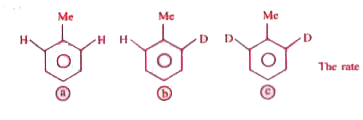 The rate of nitration will be
The rate of nitration will be