A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Van't-Haff factor (i) for this reaction is
Text Solution
|
- , van't -Hoff factor (i) for this reaction is:
Text Solution
|
- A solute dissociate in solution according to reaction 2Ato5B , If solu...
Text Solution
|
- Observe the following abbrevations pi(obs) = observed colligative prop...
Text Solution
|
- van't Hoff factor (i)
Text Solution
|
- If van't Hoff factor i=1,then
Text Solution
|
- The Van't Hoff factor (i) accounts for
Text Solution
|
- The Van't Hoff factor (i) accounts for
Text Solution
|
- Van't-Haff factor (i) for this reaction is
Text Solution
|
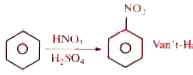 Van't-Haff factor (i) for this reaction is
Van't-Haff factor (i) for this reaction is