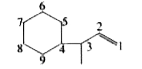
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Hydrating using H3O^(+) introduces OH majorly at carbon .................
Text Solution
|
- क्या कार्बोहाइड्रेटों को कार्बन के हाइड्रेट माना जा सकता है ?
Text Solution
|
- अभिक्रिया 2H2O hArr H3O^(+) + OH^(-) में, जल है
Text Solution
|
- On the basis of following features identify correct option. a) These e...
Text Solution
|
- Assertion : Kw increases with increase in temperature. Reason : Conc...
Text Solution
|
- Combustion reactions are majorly used as sources .
Text Solution
|
- What is the concentration of H3O^+ and OH^- ions in water at 298 K ?
Text Solution
|
- In 1 liter of water H3O^+ and OH^- Calculate the ratio of the number o...
Text Solution
|
- Which metal carbonates are majorly present in ash of plant herb ?
Text Solution
|