Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ASSAM ACADEMIC CENTRE-Chemical Kinetics -Example
- The rate of reaction triples when temperature change from 20^@C to 50^...
Text Solution
|
- Consider the reaction, 2N2O5 rarr 4NO2 + O2 The concentration of N2O5 ...
Text Solution
|
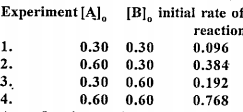
- For the chemical reaction A + 2B rarr 2C + D the experimentally determ...
Text Solution
|
- A reaction SO2Cl2 rarr SO2 + Cl2 is first order reaction with half lif...
Text Solution
|
- A certain reaction is 50% complete in 20 minutes at 300K and the same ...
Text Solution
|
- The decomposition of A into product has value of K at 4.5xx10^3s^-1 at...
Text Solution
|
- The activation energy of a reaction is 75.2 KJ mol^-1 in the absence o...
Text Solution
|
- Differentiate between order of reaction and molecularity.
Text Solution
|
- In the reaction A + B rarr Product, the initial rate becomes 4 times i...
Text Solution
|
- For the reaction R rarr P the concentration of a reactant changes from...
Text Solution
|
- In a reaction, 2A rarr Products, the concentration of A decreases from...
Text Solution
|
- For a reaction, A + B rarr Product, the rate law is given by, r = k[A]...
Text Solution
|
- The conversion of molecules X to Y follows second order kinetics. If c...
Text Solution
|
- A first order reaction has a rate constant 1.15xx10^-3 s^-1. How long ...
Text Solution
|
- Time required to decompose SO2Cl2 to half of its initial amount is 60 ...
Text Solution
|
- What will be the effect of temperature on rate constant?
Text Solution
|
- The rate of the chemical reaction doubles for an increase of 10 K in a...
Text Solution
|
- The activation energy of a reaction 2H I(g) rarrH2 + I(2(g)) is 209.5 ...
Text Solution
|
- From the rate expression for the following reactions, determine their ...
Text Solution
|
- From the rate expression for the following reactions, determine their ...
Text Solution
|