Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CHETANA PUBLICATION-Structure of atoms and nuclei-EXERCISE
- In the following table, seven elements P,Q,R,S,T,U and V (here letters...
Text Solution
|
- The radius of 3rd Bohr's orbit in hydrogen atom is 4.782 xx 10^-10 m C...
Text Solution
|
- Find the ratio of the diameters of 1st Bohr orbit to 3rd Bohr orbit.
Text Solution
|
- Calculate the linear velocity and angular momentum of an electron in t...
Text Solution
|
- Angular speed of the electron in the 1st Bohr orbit is 4.08 xx10^16 ra...
Text Solution
|
- Find the period of revolution of electron in the second orbit in the h...
Text Solution
|
- The angular momentum of the electron in the second Bohr orbit is 2.112...
Text Solution
|
- Find the energy of the electron in eV in the third Bohr orbit of the h...
Text Solution
|
- Energy of an electron in the first Bohr orbit is -13.6eV. Hence calcul...
Text Solution
|
- What is rolling kinetic energy ?
Text Solution
|
- Calculate the difference in energies of two levels between which a tra...
Text Solution
|
- An electron in the ground state jumps to 4th state by absorption of en...
Text Solution
|
- Calculate the wavelength of a microwave of frequency 8 GHz
Text Solution
|
- The Ha line of the Balmer series of hydrogen spectrumhasa wavelength 6...
Text Solution
|
- Calculate the energy radiated by the electron in the hydrogen atom dur...
Text Solution
|
- Find the series limit of Paschen series R = 1.097 xx 10^7 m^1.
Text Solution
|
- Find the ratio of the longest wavelength to the shortest wavelength in...
Text Solution
|
- If the shortest wavelength in Paschen series is 8203 A^@, find the lon...
Text Solution
|
- John Dalton's model of atom was
Text Solution
|
- Bohr's theory applicable to
Text Solution
|
- If the radius of 1st Bohr orbit in hydrogen atom is 0.5 A^@ 'then radi...
Text Solution
|
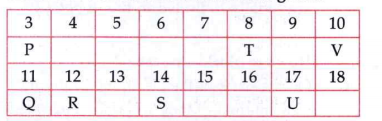 Which of these is an inert gas? Name it.
Which of these is an inert gas? Name it.