Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MAXIMUM PUBLICATION-STATES OF MATTER-EXERCISE
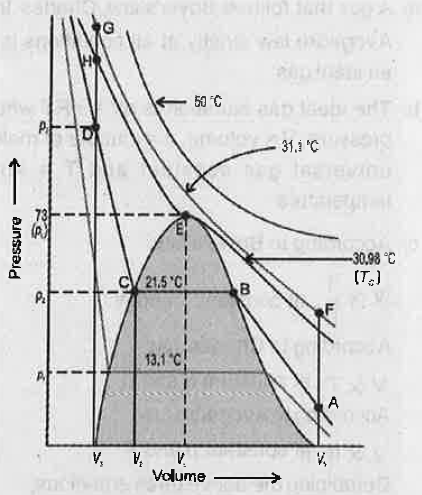
- The isotherm of carbon dioxide at various temperatures is given below:...
Text Solution
|
- What will be the minimum pressure required to compress 500 dm^3 of air...
Text Solution
|
- Using the equation of state pV=nRT show that at a given temperature , ...
Text Solution
|
- The density of a gas is found to by 5.46 g/ dm^(3) at 27° C and under ...
Text Solution
|
- Calculate the volume occupied by 8.8 g of CO2 at 31.1°C and 1 Bar pres...
Text Solution
|
- Explain the significance of van der Waal parameters
Text Solution
|
- Mercury drops are spherical in shape . a) Which property is responsi...
Text Solution
|
- How surface tension depends on temperature?
Text Solution
|
- The theory that attempts to explain the behaviour of gases is known as...
Text Solution
|
- Liquid drops attains spherical shape. Which property of liquids is res...
Text Solution
|
- How does temperature influence the viscosity of a liquid?
Text Solution
|
- Write the ideal gas eqation and mention the terms.
Text Solution
|
- It is found that real gases do not obey ideal gas equation perfectly u...
Text Solution
|
- It is found that real gases do not obey ideal gas equation perfectly u...
Text Solution
|
- In the Celsius scale, melting point of ice is 0°C . Another scale of t...
Text Solution
|
- What is the volume of ideal gas at absolute zero of temperature?
Text Solution
|
- Draw a graph showing the relationship between volume and temperature o...
Text Solution
|
- Consider a gas at 0° C. At what temperature will the volume be doubled...
Text Solution
|
- a) Name the gass law shown by the above graph.
Text Solution
|
- b) State the gas law.
Text Solution
|
- a) At 35°C and 700 mm of Hg pressure , a gas occupies a 500 mL volume ...
Text Solution
|