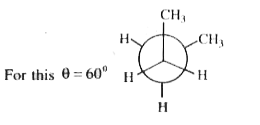
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ISOMERISM
AAKASH SERIES|Exercise PRACTICE SHEET (EXERCISE-II (LEVEL-II) STRAIGHT OBJECTIVE TYPE QUESTIONS)|29 VideosISOMERISM
AAKASH SERIES|Exercise PRACTICE SHEET (EXERCISE-II (LEVEL-II) LINKED COMPREHENSION TYPE QUESTIONS)|5 VideosISOMERISM
AAKASH SERIES|Exercise PRACTICE SHEET (EXERCISE-I (LEVEL-II) INTEGER TYPE QUESTIONS)|3 VideosIONIC EQUILIBRIUM
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (LEVEL -II PRACTICE SHEET (ADVANCED) (Integer Type Questions))|8 VideosPERIODIC CLASSIFICATION
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 3 (RECENT AIPMT/NEET QUESTIONS)|14 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ISOMERISM -PRACTICE SHEET (EXERCISE-II (LEVEL-I) STRAIGHT OBJECTIVE TYPE QUESTIONS)
- The dihedral angle between the two methyl groups in gauche conformatio...
Text Solution
|
- The dihedral angle between the two methyl groups in anti conformation ...
Text Solution
|
- Which of the following is the energy profile diagram for n-butane abou...
Text Solution
|
- Which points on the potential energy diagram represent the eclipsed co...
Text Solution
|
- Consider the following conformations of 3-Aminopropanal, amongst the g...
Text Solution
|
- The most stable conformation of ethylene glycol is
Text Solution
|
- Flagpole interaction is present in
Text Solution
|
- Which of the following is associated with Torsional strain ?
Text Solution
|
- Among the structure shown below, which has lowest potential energy?
Text Solution
|
- Increasing order of stability among the three main conformation (i.e. ...
Text Solution
|
- In which of the following has minimum torsional strain and minimum Van...
Text Solution
|
- Which of the following pairs of compounds are geometrical isomers
Text Solution
|
- The cause of cis-trans isomerism is
Text Solution
|
- Which of the following compounds exhibits geometrical isomerism?
Text Solution
|
- Which of the following is classified as Z and trans-isomer?
Text Solution
|
- Geometrical isomerism results because molecules has :
Text Solution
|
- Which of the following does not show geometrical isomerism?
Text Solution
|
- https://media.cheggcdn.com/media%2F1d8%2F1d8cc659-3b9f-4ef8-be87-3f7a6...
Text Solution
|
- Which of the following compounds exhibits geometrical isomerism?
Text Solution
|
- Which of the following order of stability is correct among these two i...
Text Solution
|