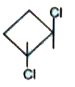
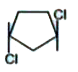
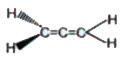
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ISOMERISM
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (LEVEL-II LECTURE SHEET (ADVANCED) LINKED COMPREHENSION TYPE QUESTIONS)|6 VideosISOMERISM
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (LEVEL-II LECTURE SHEET (ADVANCED) MATRIX MATCHING TYPE QUESTIONS)|3 VideosISOMERISM
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (LEVEL-I(MAIN) STRAIGHT OBJECTIVE TYPE QUESTIONS)|72 VideosIONIC EQUILIBRIUM
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (LEVEL -II PRACTICE SHEET (ADVANCED) (Integer Type Questions))|8 VideosPERIODIC CLASSIFICATION
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 3 (RECENT AIPMT/NEET QUESTIONS)|14 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ISOMERISM -ADDITIONAL PRACTICE EXERCISE (LEVEL-II LECTURE SHEET (ADVANCED) MORE THAN ONE CORRECT ANSWER TYPE QUESTIONS)
- Which of the following have zero dipole moment?
Text Solution
|
- Which of the following statements is/are not correct?
Text Solution
|
- Tautomerism form of this compound is/are:
Text Solution
|
- Which of the following statements regarding 2-butene is / are right
Text Solution
|
- About meso tartaric acid which of the following statements is/are not ...
Text Solution
|
- Which pair does represent isomers?
Text Solution
|
- The molecule H3C - CH = CH - underetoverset(|)(CH3)(C )H - COOH can e...
Text Solution
|
- Which of the following statements is /are incorrect about two organic ...
Text Solution
|
- In which of the following molecules chiral centre(s) is/are not presen...
Text Solution
|
- Mark out the optically active molecule :
Text Solution
|
- The structures I and II are :
Text Solution
|