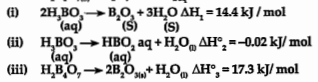Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CHETANA PUBLICATION-Chemical Thermodynamics-EXERCISE
- Calculate DelatH^@ forthe followingreaction at 298K H2B4O(7(s))+H2Oira...
Text Solution
|
- The correct thermodynamic conditions for the spontaneous reaction at a...
Text Solution
|
- A gas is allowed to expand in a well insulated container against a con...
Text Solution
|
- In which of the following entropy of the system decreases?
Text Solution
|
- The enthalpy of formation for all elements in their standard state is
Text Solution
|
- Which of the following reaction is exothermic ?
Text Solution
|
- 6.24 g of ethanol are vaporized by supplying 5.89kJ of heat.Enthalpy o...
Text Solution
|
- If the standard enthalpy of formation of methanol is -238.9 kJ mol^(-1...
Text Solution
|
- For vaporization of water at 1bar,DeltaH=40.63kJ mol^(-1)and DeltaS = ...
Text Solution
|
- Bond enthalpies of H-H, Cl-Cl and H-Cl bonds are 434 kj mol^(-1), 242 ...
Text Solution
|
- Which of the following is not a state function?
Text Solution
|
- Which of the following is not an extensive property?
Text Solution
|
- An endothermic reaction is one in which heat content of
Text Solution
|
- In a chemical reaction, work is done by the system when
Text Solution
|
- Which of the following is an intensive property?
Text Solution
|
- When a sample of an ideal gas is allowed to expand at constant tempera...
Text Solution
|
- A gas of 0.320 kJ works on its surroundings and absorbs 120 J of heat ...
Text Solution
|
- In the reaction, H2 + Cl2 rarr 2HCl DeltaH =-184 kJ, if 2 moles of H2 ...
Text Solution
|
- The enthalpies of formation of N2O and NO at 298 K are 82 and 90 kJ mo...
Text Solution
|
- For which of the following substances DeltafH^@ is not zero.
Text Solution
|
- If for a reaction, DeltaH is negative and DeltaS is positive then the ...
Text Solution
|