Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CHETANA PUBLICATION-CHEMICAL KINETICS-EXERCISE
- The rate of the first order reaction A rarr products, is 0.01 M sr1, w...
Text Solution
|
- If a graph is plotted between Ln K and 1//T for the first order reacti...
Text Solution
|
- The half life of a first order reaction is 30 min and the initial conc...
Text Solution
|
- Derive the integrated rate law for zero order reaction.
Text Solution
|
- Identify the Molecularity of following reaction- Cl(g)+Cl(g)+N(2(g)) r...
Text Solution
|
- The graph of temperature against time is
Text Solution
|
- Express the rate of the following reaction in terms of change in conce...
Text Solution
|
- Distinguish between: Order and Molecularity.
Text Solution
|
- What is the Molecualrity and order of the following examples - 2NO2+...
Text Solution
|
- Find the overall order CHCl(3(g))+Cl(2(g)) rarr CCl(4(g))+HCl(g) Rat...
Text Solution
|
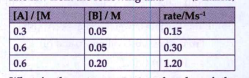
- For the reaction 2A + B rarr products, find the rate law from the fol...
Text Solution
|
- Write a note on semen.
Text Solution
|
- Draw the graph of [A]t against time for a first order reaction.
Text Solution
|
- The rate constant of a first order reaction is 6.8xx10^(-4)s^(-1). If ...
Text Solution
|
- reaction has rate constant 1.73 xx10^(-3) min^(-1) and 4.86xx10^(-3) m...
Text Solution
|
- A catalyst increases the rate of the reaction by
Text Solution
|
- The rate law for the reaction C2H4Br2+3I^- rarr C2H4+2Br^-+I3^- is rat...
Text Solution
|
- For the reaction NO2+CO rarr NO+ CO2, the rate of reaction if, experim...
Text Solution
|
- A complex reaction takes place in two steps as follows. Write its over...
Text Solution
|
- A complex reaction takes place in two steps as follows. Write its over...
Text Solution
|