Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MBD PUBLICATION-DUAL NATURE OF RADIATION AND MATTER-QUESTION BANK
- Monochromatic light of frequency 6.0 xx 10^14 Hz is produced by a lase...
Text Solution
|
- How many photon per second, on the average are emitted by the source ?
Text Solution
|
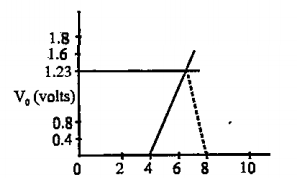
- Using the graph as in figure for stopping potential vs the incident fr...
Text Solution
|
- The velocity of a body of mass 1 kg is 1m/s. Calculate de Broglie wave...
Text Solution
|
- What is de Broglie wavelength of an electron moving with 1/(20)th of v...
Text Solution
|
- Calculate the wavelength of an electron accelerated under a p.d of 150...
Text Solution
|
- Calculate the de Broglie wavelength of a neutron of energy 0.022 eVm =...
Text Solution
|
- Calculate the energy of the neutron , if it's wavelength is 1 A^@ ?
Text Solution
|
- An electron and proton are possessing same amount of kinetic energy. W...
Text Solution
|
- Is the de Broglie wavelength of a photon of an em radiation equal to t...
Text Solution
|
- Are matter waves electro magnetic ?
Text Solution
|
- Why cannot we experience the existence of matter waves in our day life...
Text Solution
|
- What is the difference between light waves and matter waves ?
Text Solution
|
- A proton and an electron have same de Broglie wavelength, which posses...
Text Solution
|
- A photon and electron have got same de Broglie wavelength. Which has g...
Text Solution
|
- What is the dimensional formula of h/(mv) ?
Text Solution
|
- What are application of matter waves ?
Text Solution
|
- How do we define photoelectric work function and how it is related to ...
Text Solution
|
- Do all photons have same mass ? If not why ?
Text Solution
|
- Which photon is more energetic ? A red one or violet one ?
Text Solution
|