Similar Questions
Explore conceptually related problems
Recommended Questions
- Find overall order of given reaction using following experimental data...
Text Solution
|
- The air oxidation of nitric oxide is one of the reactions that contrib...
Text Solution
|
- Consider the following data for the reaction: A+B to Products Determin...
Text Solution
|
- For a reaction between A and B, the initial rate of reaction is measu...
Text Solution
|
- Use the experimental data in the table to determine was studies ...
Text Solution
|
- For a reaction 2P + Q to S , following data were collected. Calculate ...
Text Solution
|
- For a reaction 2NO(g) + 2H2(g) to N2(g) + 2H2O(g) , the following data...
Text Solution
|
- For a reaction 2NO(g) + 2H2(g) to N2(g) + 2H2O(g) , the following data...
Text Solution
|
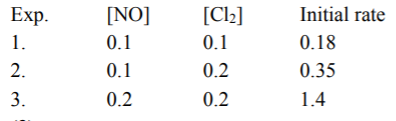
- The experimental data for the reaction : 2NO(g) +CI(2) (g) rarr 2NOCI(...
Text Solution
|