Similar Questions
Explore conceptually related problems
Recommended Questions
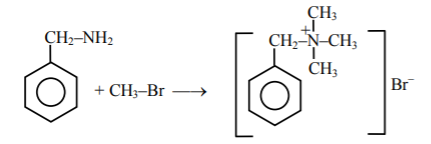
- Moles of Methylbromide required to form 23g Trimethylbenzyl ammonium b...
Text Solution
|
- The value of the expression 1-((n/1).((1+x)/(1+nx))+((n(n-1))/(1.2))((...
Text Solution
|
- Cetyl trimethyl ammonium bromide is a :
Text Solution
|
- Cetyl trimethyl ammonium bromide is a popular
Text Solution
|
- Ceric ammonium sulphate and potassium permanganate are used as oxidisi...
Text Solution
|
- Ceric ammonium sulphate and potassium permanganate are used as oxidisi...
Text Solution
|
- How many moles of ethyl bromide is required to convert ethanamine to N...
Text Solution
|
- How many moles of methyl iodide would he required to form quater...
Text Solution
|
- If (n^(n))/(n!)=(nx)/((n-1)!), x =
Text Solution
|