Text Solution
Verified by Experts
Topper's Solved these Questions
THERMODYNAMICS
AAKASH SERIES|Exercise EXERCISE - 1A|135 VideosTHERMODYNAMICS
AAKASH SERIES|Exercise EXERCISE - 1B|32 VideosTHERMODYNAMICS
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|7 VideosTHERMAL PROPERTIES OF MATTER
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (LEVEL - II) PRACTICE SHEET (ADVANCED) Integer/Subjective Type Questions|2 VideosTHERMOMETRY
AAKASH SERIES|Exercise Numerical Exercise (LEVEL- 1)|11 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-THERMODYNAMICS-Problem
- A perfect gas undergoes the following three separate and distinct proc...
Text Solution
|
- When 5 moles of an ideal gas is compressed isothermally, its volume de...
Text Solution
|
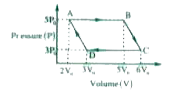
- An ideal monoatomic gas is taken round the cycle ABCDA as shown in the...
Text Solution
|
- Two moles of a gas is expanded to double its volume by two different p...
Text Solution
|
- Temperature of 1 mole of an ideal gas is increased from 300 k to 310 k...
Text Solution
|
- If a monoatomic ideal gas of volume 1 litre at N.T.P is compressed adi...
Text Solution
|
- A tyre pumped to a pressure of 6 atmosphere suddenly burst. Room termp...
Text Solution
|
- An ideal gas mixture filled inside a balloon expands according to the ...
Text Solution
|
- An ideal gas expands isothermally from a volume V(1) " to " V(2) and t...
Text Solution
|
- Three samples of the same gas A,B and C (gamma=3//2) have initially eq...
Text Solution
|
- 0.2 m^(3) of an ideal gas at a pressure of 2 Mpa and 600 K is expanded...
Text Solution
|
- Draw the P-T and V-T diagrams of an isochoric process of n moles of an...
Text Solution
|
- Draw the P-T and V-T diagrams for an isobaric process of expansion, co...
Text Solution
|
- Draw the P-T and V-T diagrams for an isothermal process, corresponding...
Text Solution
|
- At 27^(@)C , two moles of an ideal mono-atomic gas occupy a volumeV. T...
Text Solution
|
- A piston divides a closed gas cylinder into two parts. Initially the p...
Text Solution
|
- An ideal gas is compressed to half of the volume. How much work is don...
Text Solution
|
- P-V diagram of a diatomic gas is a straight line passing through origi...
Text Solution
|
- Find the molar heat capacity in a process of a diatomic gas if it does...
Text Solution
|
- A monoatomic gas undergoes a process given by 2dU+3dW=0, then what is ...
Text Solution
|