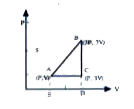
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
AAKASH SERIES|Exercise EXERCISE - 3|33 VideosTHERMODYNAMICS
AAKASH SERIES|Exercise EXERCISE - 1B|32 VideosTHERMAL PROPERTIES OF MATTER
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (LEVEL - II) PRACTICE SHEET (ADVANCED) Integer/Subjective Type Questions|2 VideosTHERMOMETRY
AAKASH SERIES|Exercise Numerical Exercise (LEVEL- 1)|11 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-THERMODYNAMICS-EXERCISE - 2
- Two moles of an ideal monoatomic get at 27^(@)C occupies a volume of V...
Text Solution
|
- A container of volume 1m^(3) is divided into two equal compartments, o...
Text Solution
|
- A cylinder with a movable piston contains 3 moles of hydrogen at stand...
Text Solution
|
- How much work to be done in decreasing the volume of and ideal gas by ...
Text Solution
|
- A gas at 10^(@)C temperature and 1.013xx10^(5) Pa pressure is compress...
Text Solution
|
- An ideal gas is taken around ABCA as shown in the above diagram. The w...
Text Solution
|
- Heat energy absorbed by a system in going through a cyclic process sho...
Text Solution
|
- P-V diagram of an ideal gas is shown in the figure. Work done by the g...
Text Solution
|
- The efficiency of a heat engine if the temperature of the source is 10...
Text Solution
|
- A Carnot engine working between 300 K and 600 K has work output of 800...
Text Solution
|
- In a mechanical refrigerator, the low temperature coils are at a tempe...
Text Solution
|
- An ideal heat engine exhausting heat at 77^@C is to have a 30% effici...
Text Solution
|
- A cannot engine extracts heat from water at 0^(@)C and rejects it to r...
Text Solution
|
- Five moles of hydrogen initially at STP is compressed adiabatically so...
Text Solution
|
- A Carnot refrigerator absorbs heat from water at 0^(@)C and gives it t...
Text Solution
|
- An ideal gas is taken through a process as shown in the figure. It abs...
Text Solution
|
- One mole of an ideal monoatomic gas undergoes a process described by t...
Text Solution
|
- The temperature inside a refrigerator is t(2).^(@)C and the room tempe...
Text Solution
|
- A refrigerator works between 4^(@)C and 30^(@)C. It is required to rem...
Text Solution
|
- The efficiency of Carnot heat engine is 0.5 when the temperature of th...
Text Solution
|