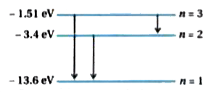
Text Solution
Verified by Experts
Topper's Solved these Questions
ATOMS
KUMAR PRAKASHAN|Exercise SECTION-B (Additional Exercises)|13 VideosATOMS
KUMAR PRAKASHAN|Exercise NUMERICAL FROM DARPAN BASED ON TEXTBOOK|10 VideosATOMS
KUMAR PRAKASHAN|Exercise SECTION-B (NUMERICALS) (Numerical From Textual Illustrations)|9 VideosALTERNATING CURRENTS
KUMAR PRAKASHAN|Exercise SECTION-D MCQs (COMPETITIVE EXAMS)|64 VideosBOARD'S QUESTION PAPER MARCH-2020
KUMAR PRAKASHAN|Exercise PART-B SECTION -C|4 Videos
Similar Questions
Explore conceptually related problems
KUMAR PRAKASHAN-ATOMS-SECTION-B (Numerical From Textual Exercise)
- The size of the atom in Thomson's model is .......... the atomic size ...
Text Solution
|
- In the ground state of .......... electrons are in stable equilibrium,...
Text Solution
|
- A classical atom based on .......... is doomed to collapse. (Thomson's...
Text Solution
|
- Choose the correct alternative from the clues given at the end of the ...
Text Solution
|
- Choose the correct alternative from the clues given at the end of the ...
Text Solution
|
- Suppose you are given a chance to repeat the alpha- particle scatterin...
Text Solution
|
- What is the shortest wavelength present in the Paschen series of spect...
Text Solution
|
- A difference of 2.3 eV separates two energy levels in an atom. What is...
Text Solution
|
- The ground state energy of hydrogen atom is –13.6 eV. What are the kin...
Text Solution
|
- A hydrogen atom initially in the ground level absorbs a photon, which ...
Text Solution
|
- (a) Using the Bohr’s model calculate the speed of the electron in a hy...
Text Solution
|
- (a) Using the Bohr’s model calculate the speed of the electron in a hy...
Text Solution
|
- The radius of the innermost electron orbit of a hydrogen atom is 5.3 x...
Text Solution
|
- A 12.5 eV electron beam is used to bombard gaseous hydrogen at room te...
Text Solution
|
- In accordance with the Bohr's model find the quantum number than chara...
Text Solution
|