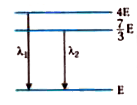
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMS
KUMAR PRAKASHAN|Exercise Section-D (Multiple Choice Questions (MCQs)) (MCQs From .DARPAN Based On Textbook) (de-Broglie.s Explanation of Bohr.s Second Postulate of Quantisation)|10 VideosATOMS
KUMAR PRAKASHAN|Exercise Section-D (Multiple Choice Questions (MCQs)) (MCQs From .DARPAN Based On Textbook) (MCQs based on Textual Illustrations and Exercise )|11 VideosATOMS
KUMAR PRAKASHAN|Exercise Section-D (Multiple Choice Questions (MCQs)) (MCQs From .DARPAN Based On Textbook) (Bohr Model of Hydrogen Atom )|21 VideosALTERNATING CURRENTS
KUMAR PRAKASHAN|Exercise SECTION-D MCQs (COMPETITIVE EXAMS)|64 VideosBOARD'S QUESTION PAPER MARCH-2020
KUMAR PRAKASHAN|Exercise PART-B SECTION -C|4 Videos
Similar Questions
Explore conceptually related problems
KUMAR PRAKASHAN-ATOMS-Section-D (Multiple Choice Questions (MCQs)) (MCQs From .DARPAN Based On Textbook) (Line Spectrum of Hydrogen Atom)
- In an atom when electrons transition from excited state to ground stat...
Text Solution
|
- The wavelength of spectrum line is inversely proportional to the ........
Text Solution
|
- The wavelength involved in the spectrum of deuterium (.(1)^(2)D) are s...
Text Solution
|
- Hydrogen atom excites energy level from n = 3 to ground state. Number ...
Text Solution
|
- Hydrogen atom emits blue light when it changes from energy level n = 4...
Text Solution
|
- What is the frequency in Hz of emitted radiation when an electron jump...
Text Solution
|
- The wavelength of first line of Balmer series in hydrogen atom is 2, t...
Text Solution
|
- Hydrogen atom from excited state comes to the ground state by emitting...
Text Solution
|
- The shortest wavelength of the spectrum line is that of electron being...
Text Solution
|
- Below are shown the energy levels for a particular atom. A photon with...
Text Solution
|
- The diagram shows the energy levels for an electron in a certain atom....
Text Solution
|
- The maximum wavelength of Brackett series of hydrogen atom is ...... [...
Text Solution
|
- The shortest wavelength in the Lyman series is 911.6Å. Then the longes...
Text Solution
|
- Which of the following lines represents the wavelength of the spectrum...
Text Solution
|
- If the minimum wavelength of Balmer series is lamda(1) and the maximum...
Text Solution
|
- Which of the following determine shortest and longest wavelengths in h...
Text Solution
|
- The difference in the frequency of series limit of Lyman series and fr...
Text Solution
|
- The energy states A, B and C having energy E(A)ltE(B)ltE(C). If the tr...
Text Solution
|
- The following energy states are shown for a atom such as hydrogen. The...
Text Solution
|
- If the hydrogen atom is initially at n = 2 state, how much work have t...
Text Solution
|