A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMS
KUMAR PRAKASHAN|Exercise Section-D -MCQs asked in AIIMS|17 VideosATOMS
KUMAR PRAKASHAN|Exercise Section-D -MCQs asked in GUJCET / Board Exam|34 VideosATOMS
KUMAR PRAKASHAN|Exercise Section-D (Multiple Choice Questions (MCQs)) (MCQs From .DARPAN Based On Textbook) (MCQs based on Textual Illustrations and Exercise )|11 VideosALTERNATING CURRENTS
KUMAR PRAKASHAN|Exercise SECTION-D MCQs (COMPETITIVE EXAMS)|64 VideosBOARD'S QUESTION PAPER MARCH-2020
KUMAR PRAKASHAN|Exercise PART-B SECTION -C|4 Videos
Similar Questions
Explore conceptually related problems
KUMAR PRAKASHAN-ATOMS-Section-D (MCQs asked in Competitive Exams) (MCQs asked in AIEEE and JEE (Main))
- An alpha particle of energy 5 MeV is scattered through 180^(@) by a fi...
Text Solution
|
- An another point charge q, thrown towards the stationary point charge ...
Text Solution
|
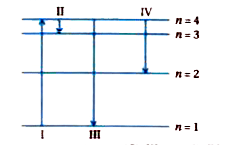
- The diagram shows the energy levels for an electron in a certain atom....
Text Solution
|
- An alpha-nucleus of energy (1)/(2)mv^(2) bombards a heavy nuclear targ...
Text Solution
|
- Which of the following transitions in hydrogen atoms emit photons of h...
Text Solution
|
- Suppose an electron is attracted towards the origin by a force (k)/(r ...
Text Solution
|
- The transistion from the state n = 4 to n = 3 in a hydrogen like atom ...
Text Solution
|
- The energy required for electron to transist from n = 1 to n = 3 in Li...
Text Solution
|
- Hydrogen atom is excited from ground state to another state with princ...
Text Solution
|
- The ionisation potential for ...... nucleus will be minimum.
Text Solution
|
- In a hydrogen like atom electron makes transition from an energy level...
Text Solution
|
- The radiation corresponding to 3 to 2 transition of hydrogen atom fall...
Text Solution
|
- Hydrogen (""(1)H^(1)), Deuterium (""(1)H^(2)), single ionised Helium (...
Text Solution
|
- Dia-atomic molecule is composed of masses m(1) and m(2) and distance b...
Text Solution
|
- As an electron makes a transition from an excited state to the ground ...
Text Solution
|
- Some energy levels of a molecule are shown the figure. The ratio of th...
Text Solution
|
- An electron from various excited states of hydrogen atom emit radiatio...
Text Solution
|
- If the series limit frequency of the Lyman series is v(L), then the se...
Text Solution
|
- The time period of revolution of an electron in its ground state orbit...
Text Solution
|
- Number of the a-particle deflected in Rutherford's a-particle scatteri...
Text Solution
|