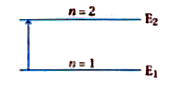A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMS
KUMAR PRAKASHAN|Exercise Section-D -MCQs asked in AIIMS|17 VideosATOMS
KUMAR PRAKASHAN|Exercise Section-D -MCQs asked in GUJCET / Board Exam|34 VideosATOMS
KUMAR PRAKASHAN|Exercise Section-D (Multiple Choice Questions (MCQs)) (MCQs From .DARPAN Based On Textbook) (MCQs based on Textual Illustrations and Exercise )|11 VideosALTERNATING CURRENTS
KUMAR PRAKASHAN|Exercise SECTION-D MCQs (COMPETITIVE EXAMS)|64 VideosBOARD'S QUESTION PAPER MARCH-2020
KUMAR PRAKASHAN|Exercise PART-B SECTION -C|4 Videos
Similar Questions
Explore conceptually related problems
KUMAR PRAKASHAN-ATOMS-Section-D (MCQs asked in Competitive Exams) (MCQs asked in AIEEE and JEE (Main))
- Energy of a hydrogen atom with principal quantum number n is shown by ...
Text Solution
|
- The total energy of an electron in the first excited state of the hydr...
Text Solution
|
- Ionization potential of hydrogen atom is 13.6 eV. Hydrogen atoms in th...
Text Solution
|
- The ground state energy of hydrogen atom is - 13.6 eV. When electron i...
Text Solution
|
- The ionization energy of the electron in the hydrogen atom in its grou...
Text Solution
|
- In a Rutherford's scattering experiment when a projectile of chargez Z...
Text Solution
|
- The energy of a hydrogen atom in the ground state is -13.6 eV. The ene...
Text Solution
|
- The wavelength of the first line of Lyman series for hydrogen atom is ...
Text Solution
|
- Out of the following which one is not a possible energy for photon to ...
Text Solution
|
- An electron in the hydrogen atom jumps from excited state n to the gro...
Text Solution
|
- The ratio of maximum wavelength of Lyman and Balmer series in hydrogen...
Text Solution
|
- Hydrogen atom in ground state is excited by a monochromatic radiation ...
Text Solution
|
- Consider 3rd orbit of Het (Helium), using non-relativistic approach, t...
Text Solution
|
- Given the value of Rydberg constant is 10^(7) m^(-1) the wave number o...
Text Solution
|
- When an o-particle of mass m moving with velocity v bombards on a heav...
Text Solution
|
- If an electron in a hydrogen atom jumps from the 3rd orbit to the 2nd ...
Text Solution
|
- If the longest wavelength of the ultraviolet region of hydrogen spectr...
Text Solution
|
- The ratio of wavelength of the last line of Balmer series and the last...
Text Solution
|
- The ratio of kinetic energy of the total energy of an electron of a Bo...
Text Solution
|
- The total energy of an electron in an atom in an orbit is -3.4 eV. Its...
Text Solution
|