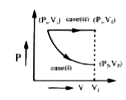
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- The initial state of a certain gas is P(i)V(i), T(i)) . It undergoes ...
Text Solution
|
- A quantity of gas occupies an initial volume V(0) at pressure p(0) and...
Text Solution
|
- The initial state of certain gas is (P(i), V(i), T(i)) . It undergoes ...
Text Solution
|
- Graphically shoe the total work done in an expansion when the state of...
Text Solution
|
- The initial state of a certain gas is (p(i),V(i),T(i)). It undergoes e...
Text Solution
|
- The initial state of certain gas (P(i)V(i)T(i)).It undergoes expansion...
Text Solution
|
- Graphically show the total work done in an expansion when the state of...
Text Solution
|
- Write expression for the work done by 1 mole of the gas in each of th...
Text Solution
|
- The initial state of certain gas is (P(i)V(i)T(i)) . It undergoes expa...
Text Solution
|