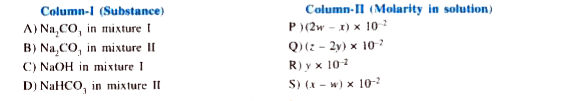Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Given two mixtures: (I) NaOH and Na(2)CO(3) and (II) NaHCO(3) and Na(2...
Text Solution
|
- In the study of titration of NaOH and Na(2)CO(3) . NaOH and NaHCO(3) ,...
Text Solution
|
- In the study of titration of NaOH and Na(2)CO(3). NaOH and NaHCO(3), N...
Text Solution
|
- In the mixture of (NaHCO(3)+Na(2)CO(3)) volume of HCl required is x mL...
Text Solution
|
- A mixture NaOH+Na(2)CO(3) required 25mL of 0.1 M HCl using phenolptht...
Text Solution
|
- 25 mL of a mixture of NaOH and Na(2)CO(3) when itrated with N//10 HCl ...
Text Solution
|
- A mixture of KOH and Na(2)CO(3) solution required 15 mL of N//20 HCl u...
Text Solution
|
- In the mixture of (NaHCO(3) + Na(2)CO(3)), volume of HCl required is x...
Text Solution
|
- If amixture of Na(2)CO(3) and NaOH in equimolar quantities when reacts...
Text Solution
|