A
B
C
D
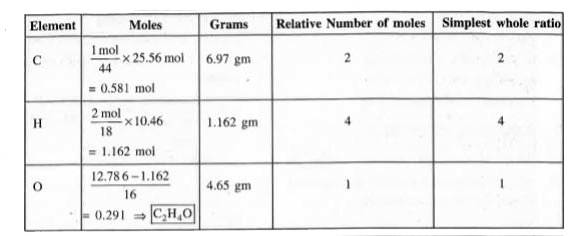
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- 12.8 gm of an organilc compound containing C(1)H(1)O and undergoes com...
Text Solution
|
- 60 gm of an organic compound containing C,H and O atoms on complete co...
Text Solution
|
- A compound contains 5 gm sulphur and 5 gm oxygen atom. The empirical f...
Text Solution
|
- 32.2 gm of an organic compound containing C,H and O when completely co...
Text Solution
|
- 3.0gms of an organic compound on combustion give 8.8 gm of CO(2) and 5...
Text Solution
|
- When hydrocarbons are burned in limited amount of air both CO and CO(2...
Text Solution
|
- 0.44 gm of organic compound C(x) H(y) O which occupied 224 ml at NTP a...
Text Solution
|
- The empirical formula of a compounds is CH(2)O . 0.25 mole of this com...
Text Solution
|
- 4.4 gms of a hydrocarbon on complete combustion produced 13.2 gms of C...
Text Solution
|