A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ALKALINE EARTH METALS-ADDITIONAL PRACTICE EXERCISE ADDITIONAL QUESTIONS
- Beryllium shows diagonal relationship with aluminium. Which one of the...
Text Solution
|
- The solubility of sulphates of alkaline earth metals in water shows th...
Text Solution
|
- The following compounds have been arranged in order of their increasin...
Text Solution
|
- A metal M readily forms its sulphate MSO(4) which is water soluble. It...
Text Solution
|
- In curing cement plasters, water is sprinkled from time to time. Which...
Text Solution
|
- Several blocks of magnesium are fixed into the bottom of ship to :
Text Solution
|
- The solubilities of carbonates decreases down ther magnesium group due...
Text Solution
|
- The substance not likely to contain CaCO(3) is
Text Solution
|
- One mole of magnesium nitride on reaction with an excess of water give...
Text Solution
|
- A sodium salt on treatment with MgCI(2) gives white precipitate only o...
Text Solution
|
- [Be(H(2)O)(4)]CI(2) on heating gives
Text Solution
|
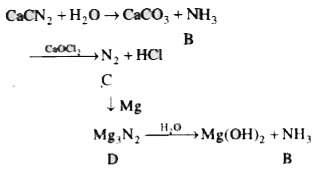
- Calcium cyanamide on hydrolysis gives a gas B which on oxidation with ...
Text Solution
|
- Alkaline earth metals form halides of the type MX(2) Which is false ab...
Text Solution
|
- A metal salt solution forms a yellow/precipitate with K(2)CrO(4) in ac...
Text Solution
|
- BeCI(2)+N(2)O(4)rarrAunderset("Vacuumn")overset(50^(@)C)rarrB overse...
Text Solution
|