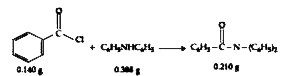Similar Questions
Explore conceptually related problems
Recommended Questions
- Consider the above reaction. The percentage yield of amide product is ...
Text Solution
|
- Calculate the molecular mass of chloroform (CHCl(3)) . (Atomic masses ...
Text Solution
|
- 7.8 g ऐक्रोलीन (C(3) H(4) O) ( mw = 56 )को उत्पन्न करने के लिए ""G 3...
Text Solution
|
- उपरोक्त अभिक्रिया पर विचार कीजिए। ऐमाइड उत्पाद की प्रतिशत लब्धि है । ...
Text Solution
|
- उपरोक्त अभिक्रिया पर विचार कीजिए जहाँ 7.8g, m-ब्रोमोबेन्जोइक अम्ल प्रा...
Text Solution
|
- In the above reaction, 3.9 g of benzene on nitration gives 4.92 g of n...
Text Solution
|
- The mole fraction of a solution in a 100 molal aqueous solution is ""x...
Text Solution
|
- A reaction of 0.1 mole of Benzylamine with bromomethane gave 23 g Benz...
Text Solution
|
- grams of 3-Hydroxy propanal (MW=74) must be dehydrated to produce 7.8 ...
Text Solution
|